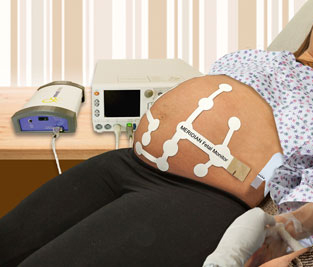
About MERIDIAN M110 Fetal Monitor
MindChild Medical has developed the MERIDIAN M110, a highly sensitive and reliable,
non-invasive fetal and maternal monitoring technology that displays fetal and maternal
heart rate as well as uterine contractions during the course of labor and delivery without
signal loss and the need for repositioning due to mother and fetal movement.
The M110 addresses multiple needs, both of the patient and clinician:
● Crowded real estate on mother’s abdomen during labor – multiple monitoring
modalities for vital signs including fetal heart rate (FHR) and uterine contractions.
● Failure to Monitor High BMI Mothers - Current standard-of-care devices have
significant limitations in obese women (>33% of pregnancies), including frequent loss
of signal, risks with invasive procedures and need for nurses to reposition multiple
devices many times a day due to loss of signal.
● A Need for High Accuracy in Fetal Heart Rate Monitoring - The MERIDIAN M110
is an advanced FHR Monitor, which uses externally placed abdominal electrodes that
acquire the fetal (fECG) signal to determine fetal heart rates - FDA 510(k) cleared.
The MERIDIAN M110 is the only non-invasive device as accurate as the invasive fetal scalp electrode (FSE) gold standard.
● A need for High Accuracy in Uterine Contraction Monitoring - The MERIDIAN M110 utilizes the same electrodes to monitor uterine contractions as well as fetal heart rate - no need for separate monitoring devices which require constant nurse manipulation to maintain accurate maternal and fetal signal reporting.
● Minimal Skin Preparation with a Light Gel Prior to Electrode Placement - The MERIDIAN M110 employs sophisticated proprietary software and algorithms, developed by the world’s leading digital signal processing experts, to analyze raw electrophysiological signals and derive the vital signs without the need for harsh skin preparation prior to electrode placement
● High and Repeatable Accuracy - The Meridian M110 demonstrated in a clinical trial: 96.7% correct fetal heart rate within +/- 5 BPM as compared to the fetal scalp electrode; 98.0% agreement of a contraction event within +/- 30 seconds as compared to the intrauterine pressure catheter; and 98.3% correct maternal heart rate within +/- 5 BPM as compared to the pulse oximeter.
Innovative Technology That Benefits All Healthcare Constituents

Patients
Providers/Physicians
Payors



- Non-invasive
- Peace of mind – continuous, elimination of gaps and missed adverse events
- No risk of infection
- Reduction of discomfort
– No repositioning required
– No tension/pressure required
- Non-invasive
- Easy to use
- Significantly increases workflow efficiency - eliminates continual repositioning
- Continuous monitoring
- Improved outcomes/quality
of care - Potential reduction of liability exposure
- Elimination of missed adverse events
- Improved outcomes/
quality of care - Lower cost
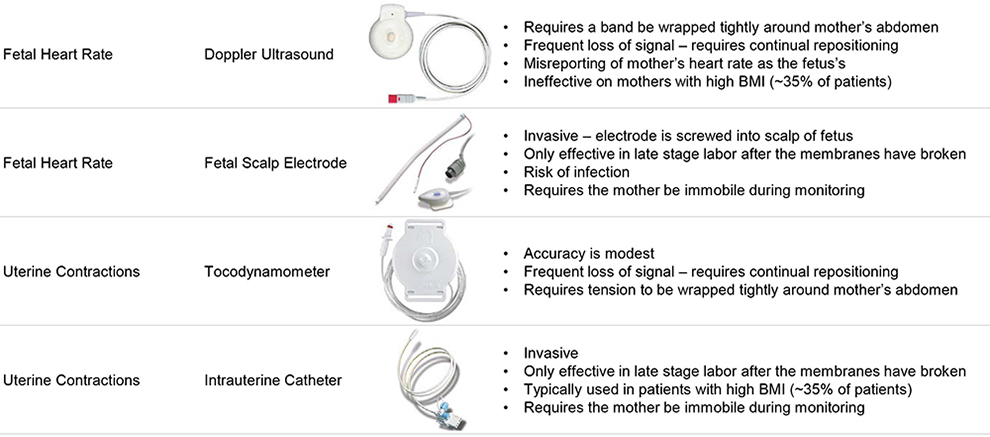
Labor and Delivery Fetal Monitoring
Vital Sign Current Monitoring Options Challenges
For more information regarding MERIDIAN M110 Monitor, please fill out the form, we will get back to you promptly.
MERIDIAN M110 Cleared by FDA for US Market

© 2021-2022 Mindchild Medical, Inc. | 1600 Osgood Street | Suite 2017 | North Andover, MA 01845 | info@mindchild.com | 978.566.9880